Building a USP 800 Compliant Compounding Clean Room
The following are two critical issues to focus on in USP-800:
- HVAC systems (negative pressure / exterior exhaust system)
- Typical layout (storage, unpacking, ante-room, and buffer room)
Why USP-800?
The reasoning behind USP-800 is to protect workers handling hazardous drugs (both non-sterile and sterile) and the environment around which they are being handled. Hazardous drugs include those used for cancer chemotherapy, antiviral drugs, hormones, some bioengineered drugs, and other miscellaneous drugs.
HVAC Systems
The HVAC system is definitely the most challenging component in the engineering of a USP-800 HD compounding facility. First, the HVAC system must achieve negative pressurization and requires dedicated exhaust ductwork to evacuate the potentially contaminated air to the outside of the building, while also minimizing the routing of exhaust equipment.
Negative pressure
The main difference between USP-797 and the compounding of hazardous drugs under USP-800 is the negative pressure requirement which aims to protect both workers and any environment which comes into contact with potential hazards. To counter cross-contamination of adjacent areas, instead of the air leaking towards the outside of the room (positive pressure), the negative pressure pulls the air towards the inside of the room, in the exhaust of the C-PEC (such as a BSC) and the low air returns of the C-SEC (buffer zone). It might seem contradictory to build a sterile ISO 7 room under negative pressure, as negative pressure pulls air particles towards the inside of the room, while ISO 7 compliance requires the room to be free of particles, however, the physics works and this is why it is so important that the HVAC system be designed correctly. Negative pressure is achieved by drawing air out of the room by means of an exhaust fan (as opposed to blowing air in with a supply fan).
Exterior exhaust
Another important aspect of USP-800-compliant facilities is that the air must be evacuated outside (no recirculation). In theory, it might look simple but depending on the location of the compounding facility within a building, this is sometimes very complex. For example, for pharmacies on the lower floor of a multiple story building, it can easily become a headache to find the optimal way to get the air outside on the roof. Hence, exterior exhaust is a tricky, and potentially costly and complicated, aspect of a USP-800 compliant compounding facility.
The HD exhaust system must lead to a roof-mounted fan location discharging at a minimum of 10 feet above the roof level and 30 feet away from fresh air intakes (standard distances – please check your local building code). Routing the hazardous exhaust throughout the existent facility sometimes includes passing HD ducts through rated fire and smoke walls. When a hazardous exhaust duct penetrates a rated area, the duct requires enclosures in a fire-resistant rated shaft. Constructing a rated-shaft enclosure around a duct can add significant cost to a project. Other regional and local regulations may apply.
Our team’s tips!
In order to maintain the negative pressure, the room runs on 100% fresh air intake which represents not only a lot of air, but additional equipment and cost to condition that air in summer and heat it in winter. Since this consumes a lot of power, make sure you have sufficient power in your building. In some cases, a heat exchanger could be used to minimize power consumption.
The Typical Compounding Cleanroom Design
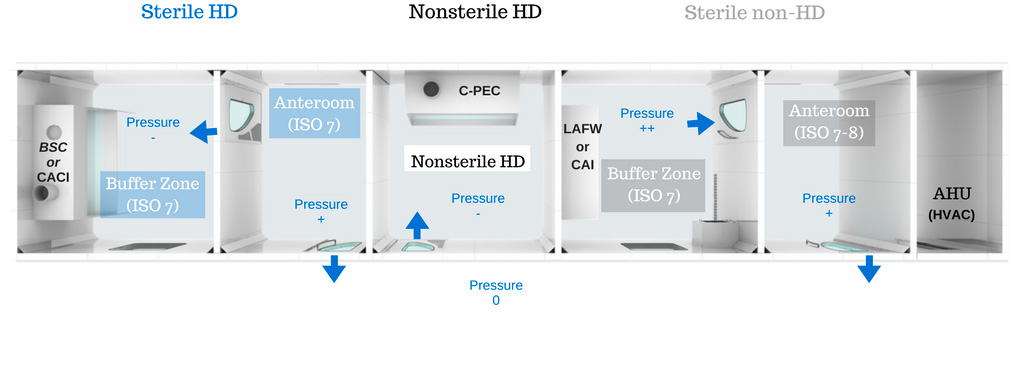
Appendix 2 of USP-800 recommends some optimal designs for HD compounding areas. Based on your type of compounding (sterile HD and/or sterile non-HD), your activities and processes will determine if you need one or several anterooms, one or several unpacking and storage rooms, etc. For example, you can share the anteroom between sterile HD and sterile non-HD but if you have the space and you compound on a regular basis, you might prefer two separate anterooms for a better workflow. You may want to seek the help of professionals to define the most appropriate layout for your needs while ensuring that your new facility is compliant.
Designated areas:
- Receipt and unpacking of HD
- Storage of HDs
- Nonsterile HD compounding
- Sterile HD compounding (+ ante-room)
Another major difference from USP-797 is that USP-800 will often require more square footage since you need an additional storage room for HD. Hazardous drugs cannot be stored with non-HD ones.
Receipt and unpacking of HD
HD must not be unpacked from their shipping containers in sterile compounding areas or positive pressure areas (such as the pharmacy). They must be unpacked in a neutral or negative pressure area. Therefore, the unpacking cannot be done in the negative pressure HD buffer room because it is a sterile environment. There are no clearly defined air change rates or pressure differential requirements for the receipt and unpacking areas.
Storage of HDs / storage room
HD must be stored in a negative pressure buffer room with at least 12 air changes per hour (ACPH) and the room must be externally vented. No pressure differential is specified. Additionally, HD and non-HD cannot be stored together. Sterile and non-sterile HDs may be stored together, but only sterile HD can be stored in the negative buffer room (sterile). If a refrigerator is placed in the negative pressure buffer room, an exhaust for its compressor is also recommended.
Our team’s tips!
USP-800 states that storage of sterile HD can be done in the clean room (buffer zone). However, you should consider that by doing so, to have access to the drugs, you will have to go inside the buffer zone (and gown up and down every time). This may not be the most efficient process if you sell a high volume of drugs on a regular basis. Storing them in an adjacent, dedicated storage room may be more effective for high volume pharmacies even if it requires an additional room and therefore increases initial costs.
Containment Segregated Compounding Area (C-SEC)
Non-Sterile HD Compounding (USP-800)
Non-sterile HD compounding must be conducted in a negative pressure enclosed area with a minimum of 12 air changes per hour and the room must be externally vented. You do not need an anteroom for non-sterile HD compounding.
C-SEC requirements
- Externally vented
- 12 ACPH
- Negative pressure between 0.01 and 0.03 inches of water
Sterile HD Compounding (USP-797 + USP-800)
ISO 7 Ante-area or anteroom
(the transition area between the unclassified area and the room containing the C-PEC)
- 30 ACPH minimum to reach an air quality of ISO 7 or better
- Positive pressure of 0.02 inches of water relative to all adjacent unclassified areas
- A cleanroom-specific sink at least 1 meter away from the entrance of the buffer room to avoid contamination of HD buffer room
ISO 7 Buffer room / clean zone (C-SEC)
- 30 ACPH minimum (to reach ISO 7)
- Externally vented
- Negative pressure between 0.01 and 0.03 inches of water
- Physically separated
Both the buffer room (through the low wall air return) and the C-PEC are externally vented.
Compounding both HD sterile and non-sterile in the same buffer room
Compounding both HD sterile and non-sterile in the same room is accepted in the regulation if the following conditions are met:
- C-PECs are placed at least a meter apart
- Room maintains ISO 7 classification
- Particle-generating activities must be stopped when sterile compounding is in process.
Even though the above is accepted, it is recommended to use a separate room for HD sterile and non-sterile compounding. It is also unpractical as well as challenging to keep a room sterile while non-sterile processes occur in the same room.
Need help with the design of your cleanroom?
DISCLAIMER
The information, statements, opinions and analyses provided by MECART in this document is designed to provide helpful and educational information on the subjects discussed. The views and opinions expressed herein constitute the judgment of the authors and are subject to change without notice. MECART has made every reasonable effort to ensure the accuracy of all the information contained in this document. However, MECART makes no representations or warranties whatsoever whether express or implied as to the accuracy, validity, correctness, currentness, completeness, suitability for any purpose of such information. Any project is different in nature and the statements provided herein shall not be deemed appropriate or relied upon for any specific application or project without subsequent analysis, evaluation, verification and assessment. MECART hereby declines any liability for any error, damage, loss, injury or other consequence which may arise from use in any manner of any information contained in this document. The advice of a competent professional should be sought before entering in any project.