Starting a Sterile Compounding Pharmacy

Considerations in Cleanroom Design
If you’ve decided to launch your sterile compounding pharmacy, but you don’t know anything about how to build the cleanroom, you can trust Mecart to help. Mecart cleanrooms are recommended by pharmacy regulatory inspectors. We have the know-how to build your sterile compounding cleanroom and we guarantee it will be compliant with required norms. In the past few years, we’ve been involved with several pharmacists who were looking for sterile compounding cleanrooms and we know how challenging a project like this can be.
In hopes of making things clearer to you, here are some insights about cleanroom design for sterile compounding pharmacies.
1) Be aware of the applicable norms, standards and regulations
2) The typical layout for sterile compounding
3) Pressure differential
4) Heating, ventilation and air conditioning system (HVAC)
—> You might also like this article: Building a USP 800 Compliant Compounding Space
1) Be aware of the applicable norms, standards and regulations
Which norms, standards, and regulations must you comply with? This is one of the first questions we ask our clients when they call for a cleanroom quote. Roughly speaking, there are three main aspects that determine which regulations apply to your compounding facility: the type of preparations that will be compounded (sterile or non-sterile, hazardous or non-hazardous); the geographic market in which you will operate, both provincial/state and national (Canada, USA); and also the geographical markets in which your compounded preparations will be sold.
What type of products will be compounded?
- Non-Hazardous Sterile Compounding Preparations
- Hazardous Sterile Compounding Preparations
- Hazardous Non-Sterile Compounding Preparations
- Non-Hazardous Non-Sterile Compounding Preparations
Although it is not recommended, it is possible to compound both hazardous and non-hazardous preparations in two adjacent clean rooms sharing the same anteroom and support zone, but strict conditions must be respected. Read the applicable norms for more information.
The most common regulations
- USP-797
- USP-800
- ISO 14644-1:2015
- NAPRA
- Your province/state pharmacy regulatory authority (eg. Ontario College of Pharmacists, College of Pharmacists of British Columbia, etc.)
In the following article, the focus will be on non-hazardous sterile compounding.
2) The typical layout for sterile compounding
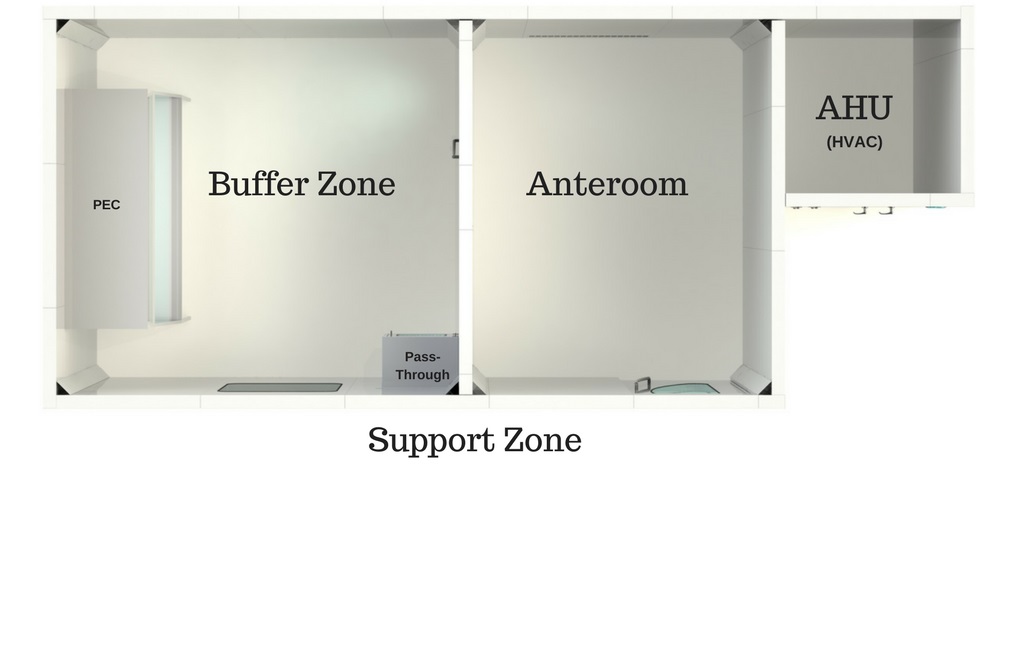
- Buffer Zone / Clean Room (ISO 7)
The actual clean room or buffer zone/area is equipped with workstations. This is where the primary engineering control (PEC) (such as LAFW, BSC, CAI and CACI) is installed. To reduce the risk of introducing contaminants, the clean room must be isolated from the rest of the pharmacy as well as from unclassified areas. Walls, pass-throughs and doors serve to separate the areas. ISO Class 7 air quality must be maintained under dynamic operating conditions. To allow the pharmacist to observe activities being conducted inside, one or more observation windows must be installed. These windows are meant to reduce the non-essential back-and-forth entries into the control areas, as well as offering safety factors, (to be able to see what is going on inside the cleanroom in case of an emergency) and also for your employees’ comfort, creating a more enjoyable, and thus productive work environment. Imagine being trapped in a room with no windows for hours on end!
- Anteroom (ISO 7 or 8)
The anteroom, also called ante-room, ante-area or antechamber, is a closed passage between the clean room and the support zone in which technicians perform support tasks (gowning, hand and forearm hygiene, labelling, etc.). It is equipped with two doors with a closing system that allows to open only one door at a time (interlock). It is actually a transitional space between the unclassified support zone and the clean room. The anteroom is usually equipped with a sink, cabinets and a bench, but equipment and activities in this area must be kept to a minimum. The anteroom can be engineered as an ISO 7 or ISO 8 environment depending on the risk level (HD or non-HD) of the sterile products being prepared in the critical area. Everything that enters the clean room must be taken through the anteroom.
- Support Zone
The support zone is not mentioned in every regulation. It is an unclassified zone that may be enclosed or not, and is used for material storage, validation, entry of prescriptions, etc.
- Area for unpacking and storing (HD products)
For hazardous product compounding, an unpacking and storing zone in a properly ventilated room with air being exhausted to the exterior may be required.
Our team’s tips!
You must define the mechanical, electrical and plumbing requirements of the sterile compounding facility. The mechanical aspects focus on heating, cooling and ventilation, the electrical aspects focus on providing power to all outlets and appliances and the plumbing aspects focus on the delivery of water and the draining of waste water. Cleanrooms usually have their own electrical panel. It is important to ensure that the building can supply the electrical power. The cleanroom HVAC (cooling and humidifying) and the anteroom’s sink require drainage and water supply.
Need help with the design of your cleanroom?
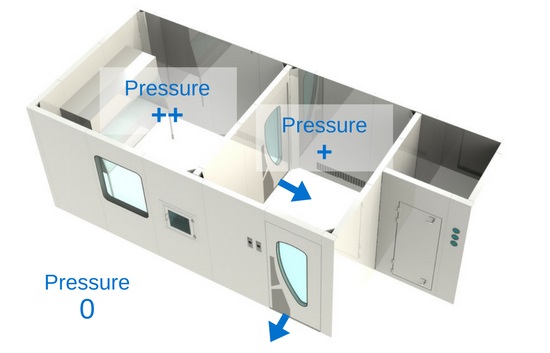
3) Pressure differential
Cleanrooms are held in positive pressure, except when dealing with the compounding of hazardous preparations, which must be held in negative pressure. The most important thing between these three zones is the pressure differential. It is crucial that the pressure gradient of the clean zone be greater (at least 5 Pa) than the anteroom’s, and that the anteroom’s pressure gradient be greater (at least 5 Pa) than the support zone’s. Positive pressure will make the air flow out of the room instead of in. What this means is that the air in the clean room will have a tendency to leak out of the room, instead of in, thus preventing unfiltered air or air particulates from entering. That being said, in a hazardous compounding facility, it is the opposite: the pressure is negative in order to prevent air that might be contaminated by hazardous products from escaping the room. Therefore, the negative pressure pushes the air towards the “clean zone”.
Our team’s tips!
HVAC for cleanrooms are different from conventional ones. The HVAC system (cleanroom ventilation and filtration) must be specified by HVAC cleanroom specialists in order to choose and engineer the appropriate system and maintain the required parameters (pressure differential, temperature, relative humidity).
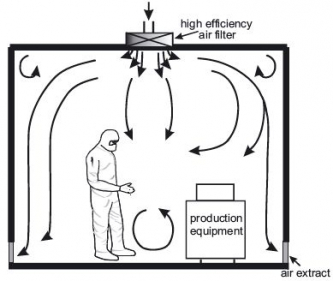
4) Heating, ventilation and air conditioning system (HVAC)
The HVAC system is the most important part of a cleanroom since it controls the supply of clean air to the cleanroom. The air intake comes from the ceiling, and is filtered through HEPA filters. The new air travels down the room, pushing the contaminated air to exits through return air intakes at the bottom of the walls. The air conditioning system is important for the employees’ comfort since wearing personal protective equipment can be quite hot. The air changes per hour (ACPH) must be of at least 30 in the clean room and of 20 ACPH in the anteroom, but may have to be greater depending on the size of the room, the number of people working inside, etc.
Our team’s tips!
- Even though regulations recommend 30 changes per hour for the clean room and 20 for the anteroom, we always design our cleanroom HVAC with a slightly higher air changes per hour, for example 40 ACPH (instead of 30) for the clean room and 30 ACPH (instead of 20) for the anteroom. This higher rate gives you time to notice a problem and fix it without having to stop operations inside the cleanroom. For example, if a HEPA filter gets dirty, the cleanroom’s higher ACPH will compensate and your cleanroom will remain compliant, giving you time to replace it. Another common example is if you decide to make small changes to the configuration inside your cleanroom. With higher air changes per hour, the air quality is less likely to be affected by the new configuration. Finally, to err is human, therefore even though you have a strict operating protocol, a higher ACPH can also compensate for a human mistake.
- Another important aspect in cleanroom design and which is often forgotten is humidity. Most regulations do not mention anything about this functional parameter, however it is important for your employees’ comfort. From our experience, the clean room tends to become very dry. For this reason, we often suggest our clients to install a humidifier in their cleanrooms. You can be sure your employees will be thanking you for it.
DISCLAIMER
The information, statements, opinions and analyses provided by MECART in this document is designed to provide helpful and educational information on the subjects discussed. The views and opinions expressed herein constitute the judgment of the authors and are subject to change without notice. MECART has made every reasonable effort to ensure the accuracy of all the information contained in this document. However, MECART makes no representations or warranties whatsoever whether express or implied as to the accuracy, validity, correctness, currentness, completeness, suitability for any purpose of such information. Any project is different in nature and the statements provided herein shall not be deemed appropriate or relied upon for any specific application or project without subsequent analysis, evaluation, verification and assessment. MECART hereby declines any liability for any error, damage, loss, injury or other consequence which may arise from use in any manner of any information contained in this document. The advice of a competent professional should be sought before entering in any project.